Encouraging Results from the Recent Phase II Clinical Trial of Our Topical Non-Opioid Pain Medication
Vapogenix has announced results of the Phase II Clinical Trial of our lead product, VPX638, which demonstrated rapid onset and sustained duration of pain relief and reduced opioid use for patients with painful wounds.
The lead product, VPX638 (sevoflurane), for topical treatment of wound pain
Vapogenix has announced results of the Phase II Clinical Trial of our lead product, VPX638, which demonstrated rapid onset and sustained duration of pain relief and reduced opioid use for patients with painful wounds.
The lead product, VPX638 (sevoflurane), for topical treatment of wound pain
- Phase 2 study demonstrated a profound, rapid and long-lasting analgesia, and reduced opioid use
- VPX638 fulfills clear unmet medical needs in this large population (10M+ US Medicare patients)
- Pain from wounds is inadequately treated with existing options
- Opioids and NSAIDs are frequently insufficient and have risk of serious side effects
- Topical analgesics have slow onset, limited effectiveness, not for chronic use, not approved for use in US
- Provides remarkable and rapid reduction in pain
- No systemic exposure
- Is opioid sparing: could reduce/eliminate opioid side effects and abuse
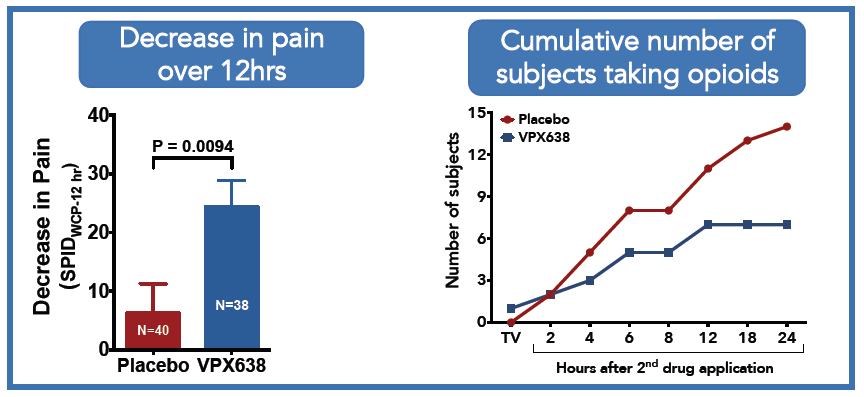
Clinical Data from Phase II Wound Study (N=78)
VPX638 decreased pain and reduced opioid use
VPX638 decreased pain and reduced opioid use
Encouraging Phase II results will inform design of the Phase III study
About the Clinical Trial
Vapogenix recently completed Phase II Clinical Trial was conducted in Australia, to determine the analgesic efficacy, safety and tolerability of its lead product, VPX638, administered topically to patients with painful wounds.
Vapogenix recently completed Phase II Clinical Trial was conducted in Australia, to determine the analgesic efficacy, safety and tolerability of its lead product, VPX638, administered topically to patients with painful wounds.
- Study was randomized, double-blind, and placebo-controlled
- Indication: Painful wounds
- Status: Completed
- 8 sites in Australia